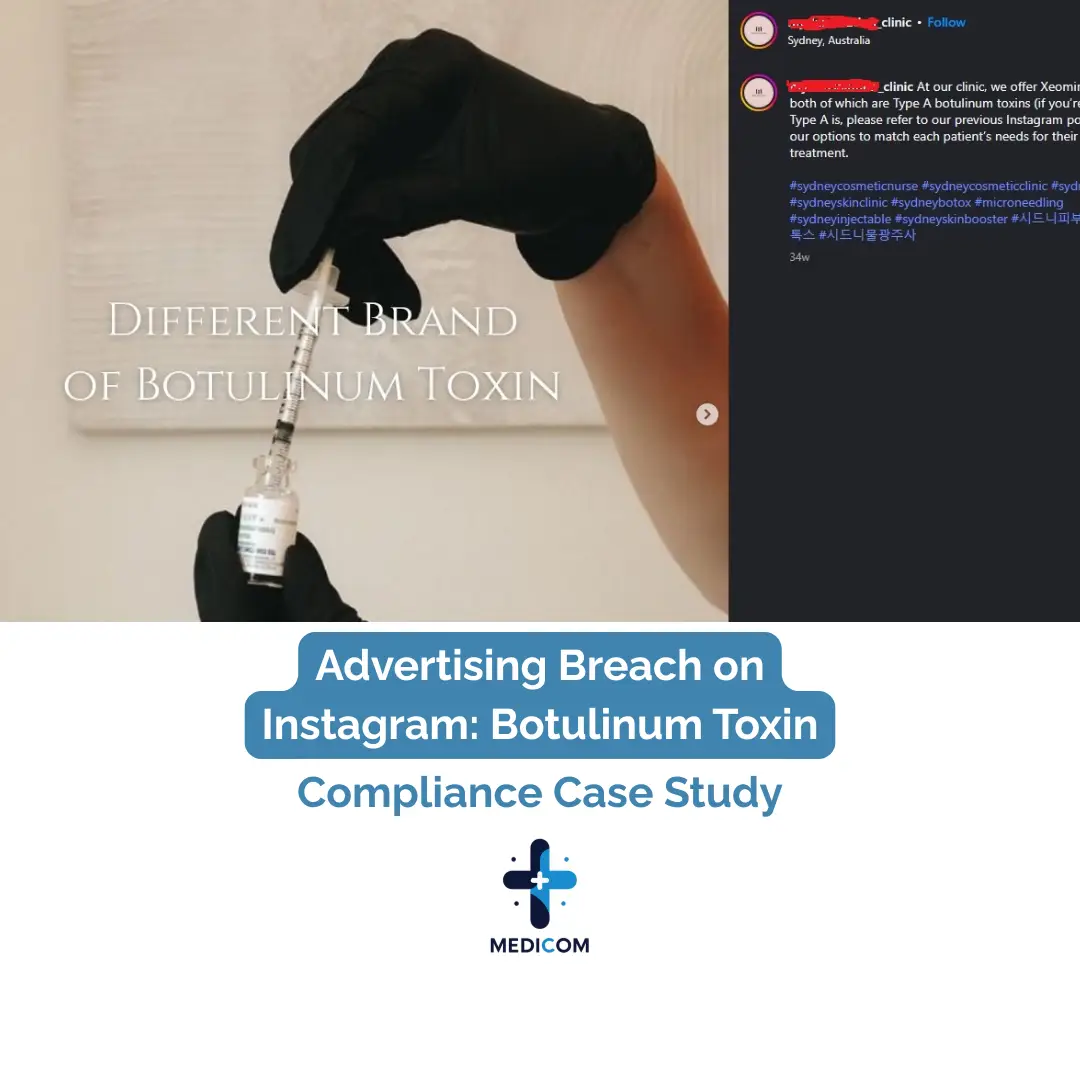
AHPRA Compliance Audit – Instagram Post (Botulinum Toxin Brands)
As part of a series of posts, and intended for educational purposes only, we highlight some actual posts seen and discuss the potential breaches or even best practice seen in the post. This is not intended to shame but to drive genuine improvement as we all come to grips with the new guidelines and how they build upon the existing guidelines.

Asset Audited: Instagram post
Date of Review: 10 July 2025
Platform: Instagram
Visual Content: A hand drawing solution into a syringe with the text: “Different Brand of Botulinum Toxin”
Caption: Refers to Xeomin and Letybo by name, describes both as “Type A botulinum toxins,” and states the clinic tailors treatment to each patient’s needs.
Hashtags: #sydneybotox, #sydneyinjectable, #cosmeticnurse, etc.
Practitioner or clinic name: Redacted
Key Compliance Breaches Identified:
Component
Description
Breach Type
Guideline Reference
Mention of Schedule 4 Drugs (Xeomin and Letybo)
The post explicitly names two prescription-only botulinum toxin brands.
🔴 Breach – TGA Code: Direct advertising of prescription medicines is prohibited.
TGA Code of Advertising, s.42DL(1)(f) of the Therapeutic Goods Act 1989
Visual Implication of Injection
The syringe being filled visually reinforces the idea of treatment with an S4 product. This is high-risk imagery.
🔴 High-risk promotional content under AHPRA’s updated non-surgical cosmetic procedure guidelines.
AHPRA Guidelines – Non-Surgical Cosmetic Procedures (Sep 2025), Visual Content Warnings
Use of Hashtag “#sydneybotox”
Even though “Botox” is not mentioned in the main text, its inclusion in hashtags constitutes an advertisement of a prescription-only substance.
🔴 Breach – TGA + AHPRA
TGA Code, AHPRA Advertising Guidelines s.6.2
Lack of Risk Disclosure or Disclaimers
No reference to risks, suitability, or side effects for botulinum toxin.
🟠 Breach of informed advertising standards.
AHPRA Do’s and Don’ts for Non-Surgical Cosmetic Procedures
Vague Claims of “Tailored Treatment”
Suggests medical judgment and benefit, yet lacks specifics or clinical justification.
🟠 Risk of misleading claims, especially when not contextualised.
AHPRA Guidelines for Advertising a Regulated Health Service, s.6.2.1
Compliance Summary
This Instagram post breaches AHPRA and TGA advertising laws due to the following:
Direct naming of prescription-only medications (Xeomin, Letybo)
Hashtag use implying prescription product advertising (#sydneybotox)
No mandatory disclaimers or risk information
Use of medical imagery (injectable preparation) that glamorises or visually promotes S4 treatments
Recommendations for Rectification
Immediately remove brand names (e.g. Xeomin, Letybo) from all public-facing ads, including social and hashtags.
Avoid imagery showing syringes or substances being prepared unless it’s part of a compliant educational or professional context.
Replace hashtags like
#sydneybotoxwith neutral alternatives (e.g.,#wrinkletreatment, only if contextually safe).Include appropriate disclaimers about risk, outcomes, and patient suitability if discussing any treatment with potential clinical risk.
If educational, consider reworking this post into a “Practitioner Insight” content series with a caption structured around informed consent and practitioner responsibility, without breaching direct advertising rules.
Lessons For Clinics
If you’re an AHPRA-registered nurse or doctor, your social content is legally regulated—even when posted to Instagram or TikTok
Naming drugs or using hashtags like “#botox” may appear minor but can trigger regulatory complaints and penalties
Always treat cosmetic marketing as healthcare advertising if it involves S4 medications, medical procedures, or regulated health practitioners
Protect Your Practice
At Medicom Compliance Auditing, we help clinics across Australia stay compliant with AHPRA, TGA, AMA, and ACCC advertising standards. Our audits identify risk, educate staff, and protect your clinic’s reputation.
📩 Need your Instagram or website content reviewed?
Book a compliance audit or contact us for an educational in-clinic workshop.